6 Finch Analysis
6.1 R scripts
6.1.1 Create a script
Until now, you have run commands by typing them into the console. One of the benefits of R over programs such as Excel, however, is the ability to save your commands in the form of written. Enter the script! A script is simply a text file containing commands for R to execute.
Now create a new script file:
- Click the New File button on the toolbar (leftmost button with a green plus sign) and choose R Script, or choose File > New File > R Script
- A new pane will open above your Console pane. This is called the Source pane.
- Immediately save your file by clicking on the blue floppy disk icon.
- Give it a memorably name like finch analysis. RStudio will automatically add the .R file extension.
6.1.2 Run code from a script
Up to this point, you have been typing code into the console to run it. This works, but it has two drawback:
- You can make typos
- If you want to run code again, you have to type it again.
From now on, use this new set of steps instead:
- Copy code from the browser. This reduces typos.
- Paste into the R script.
- Run code from the R script
The main caveat is to be careful to copy all code, especially closing parentheses ) at the end of code chunks.
Now that you have a script, try copying and pasting this code into it. For example:
sqrt(9)To run the code, place your cursor on the same line as the code as click Run on the Source pane toolbar, then Run Selected Lines. Or just hold down the Command key and press the Return key. This is called a keyboard shortcut, and is written as Cmd+Return.
When you run the line of code, RStudio copies it to the Console pane and executes it.
Now that you’ve tried it, you can delete sqrt(9) from your script before you move on.
6.2 R packages
In R, users share code with each other in the form of packages, each of which is a bundle of code, data, and other files. While R includes some basic functionality in it’s “base” packages, you often need to work with other packages when doing any data analysis.
In this tutorial you will use a group of packages collectively referred to as the tidyverse.
Before you can use a package, you need to download and install it. In Posit Cloud, we have already done this step for you by running install.packages("tidyverse") so you can skip this step.
If you are using RStudio Desktop, you will need to install the package first. In which case, you can either run the code above or use the Packages tab.
6.3 Load the packages
Below you will see two boxes, one with comments and code, and the next with example console output. The console output is just there to show you what to expect to appear in the console when you run the given code. Console output in this tutorial always appears in a box below the code, and has two pound signs before each line like this: ##
Copy the code in the first box below (including the comments) and paste it into your script.
If you still have the code sqrt(9) in your script, feel free to delete it.
# load packages -----------------------------------------------------------
# run every time you restart RStudio
library(readxl) # load readxl, for reading Excel files
library(tidyverse) # load tidyverse, for working with datasetsNow run BOTH lines of code
Put your cursor on the first line and press Cmd+Return to run it. The cursor automatically moves to the next line, so you can press Cmd+Return again to run the next line.
You will see a warning and some messages in the Console tab, but those can be safely ignored.
You must run the library() commands each time you start RStudio. That’s why it’s nice to keep that code at the very top of your script.
6.4 Read the data
Now that you have a script and have loaded the necessary packages, you’re ready to get coding!
To read the data, you will use a function named read_excel(). Here we run that function and assign the results to an object names finches_data.
# read data ----------------------------------------------------------------
# read the finches data
finches_data <- read_excel("finches-data.xlsx")If you get an error at this point (red text in the console output) see the two warnings below.
Warning If you didn’t run the two library() commands, then when your run read_excel() you will get an error that says:
Error in read_excel() : could not find function "read_excel"
To solve this error, run the two library() commands and then retry reading the data.
6.5 Inspect the data
To look at an object you have just created, print it to the console window by typing its name and running it:
# print the results in the console
finches_data#> # A tibble: 100 × 12
#> band species sex first_adult_year last_year outcome weight wing tarsus
#> <dbl> <chr> <chr> <dbl> <dbl> <chr> <dbl> <dbl> <dbl>
#> 1 9 Geospiza f… unkn… 1975 1977 died 14.5 67 18
#> 2 12 Geospiza f… fema… 1975 1977 died 13.5 66 18.3
#> 3 276 Geospiza f… unkn… 1976 1977 died 16.4 64.2 18.5
#> 4 278 Geospiza f… unkn… 1976 1977 died 18.5 67.2 19.3
#> 5 283 Geospiza f… male 1976 1977 died 17.4 70.2 19.3
#> 6 288 Geospiza f… unkn… 1976 1977 died 16.3 71.2 20.3
#> # ℹ 94 more rows
#> # ℹ 3 more variables: beak_length <dbl>, beak_depth <dbl>, beak_width <dbl>The first thing you notice is that this object is not a vector like the objects you were working with before. Instead, it’s a tibble, which is an object that stores tabular data.
The first line of the output gives the data type (tibble) and dimensions of the data (number of rows x number of columns). In the tidyverse, columns are referred to as “variables”.
The first 10 rows are shown, as well as the first few variables. How many variables depends on how wide your Console window is.
For each variable, it’s name is given, followed by the type of data. Most of the time we worth with either numeric data (<dbl>) or text data (<chr>).
If there are more than 10 rows, or more variables than can fit on the screen, there will be a sentence at the end saying “… with xx more rows, and x more variables”, followed by a list of the unshown variables.
A quick way of seeing the first few observations for all variables is to use the glimpse() function:
# take a quick look at all the variables in the dataset
glimpse(finches_data)#> Rows: 100
#> Columns: 12
#> $ band <dbl> 9, 12, 276, 278, 283, 288, 293, 294, 298, 307, 311, 3…
#> $ species <chr> "Geospiza fortis", "Geospiza fortis", "Geospiza forti…
#> $ sex <chr> "unknown", "female", "unknown", "unknown", "male", "u…
#> $ first_adult_year <dbl> 1975, 1975, 1976, 1976, 1976, 1976, 1976, 1976, 1976,…
#> $ last_year <dbl> 1977, 1977, 1977, 1977, 1977, 1977, 1977, 1977, 1977,…
#> $ outcome <chr> "died", "died", "died", "died", "died", "died", "died…
#> $ weight <dbl> 14.50, 13.50, 16.44, 18.54, 17.44, 16.34, 15.74, 16.8…
#> $ wing <dbl> 67.00, 66.00, 64.19, 67.19, 70.19, 71.19, 67.19, 68.1…
#> $ tarsus <dbl> 18.00, 18.30, 18.47, 19.27, 19.27, 20.27, 17.57, 18.1…
#> $ beak_length <dbl> 9.20, 9.50, 9.93, 11.13, 12.13, 10.63, 9.93, 11.33, 9…
#> $ beak_depth <dbl> 8.3, 7.5, 8.0, 10.6, 11.2, 9.1, 9.5, 10.5, 8.4, 8.6, …
#> $ beak_width <dbl> 8.1, 7.5, 7.6, 9.4, 9.5, 8.8, 8.9, 9.1, 8.2, 8.4, 8.5…If you want to see the entire dataset, click the name of the data object in the Environment tab, or run View(finches_data). This will open a handy data viewer window. Once you are done looking at the data, close the window and continue.
6.6 Plot a histogram
The first step in any data analysis is always to plot the data.
Because we want to compare means between two groups, a useful plot is a histogram, which shows how often each value (or range of values) was observed.
Plotting in the tidyverse is most easily accomplished using the ggplot() function and its associated helper functions.
6.6.1 Basic histogram
To plot a basic histogram of the beak_length variable, use the following code. Note the use of a + after the ggplot() function. Copy this code to your script and run it. RStudio is smart enough to recognize that it should all be run together, so putting your cursor on any of these lines and typing Cmd+Return will run all the lines at once.
# histogram ---------------------------------------------------------------
# histogram of beak length
ggplot(
data = finches_data, # use the finches dataset
mapping = aes(x = beak_length) # put beak length on the x axis
) +
geom_histogram(bins = 14) # add the histogram, use 14 bins
You can plot histograms of other variables by changing the x = part of the code.
You can also experiment with different numbers of bins. The goal is to have enough that you can see a hump-shaped distribution, but not so many that you have too many bins with few or no observations. Try plotting a histogram with 50 bins, and another with 3 bins. Not very informational, are they?
6.6.2 Add facets
Sometimes we want to see how the distribution of a variable varies among different subsets of the data. For example, to answer our question we want to see how beak length varies between birds that did or did not survive the drought. One easy way to do this is with the facet_wrap() function in ggplot. Note that you have to add a + operator after the geom_histogram() line.
# histogram of beak length, grouped by survival
ggplot(
data = finches_data, # use the finches dataset
mapping = aes(x = beak_length, # put beak length on the x axis
fill = outcome) # fill sets the color of the boxes
) +
geom_histogram(bins = 14) + # add the histogram, use 14 bins
facet_wrap(~ outcome, ncol = 1) + # outcome is the grouping variable
guides(fill = "none") # don't show a legend for fill color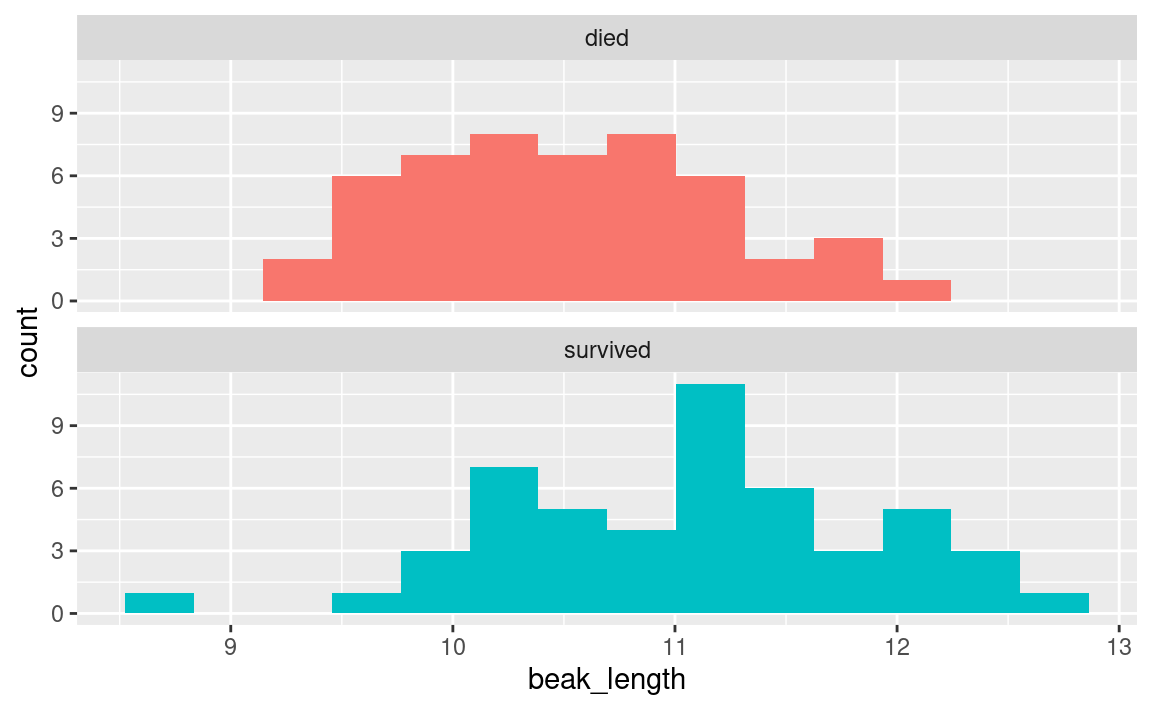
6.6.3 Add labels
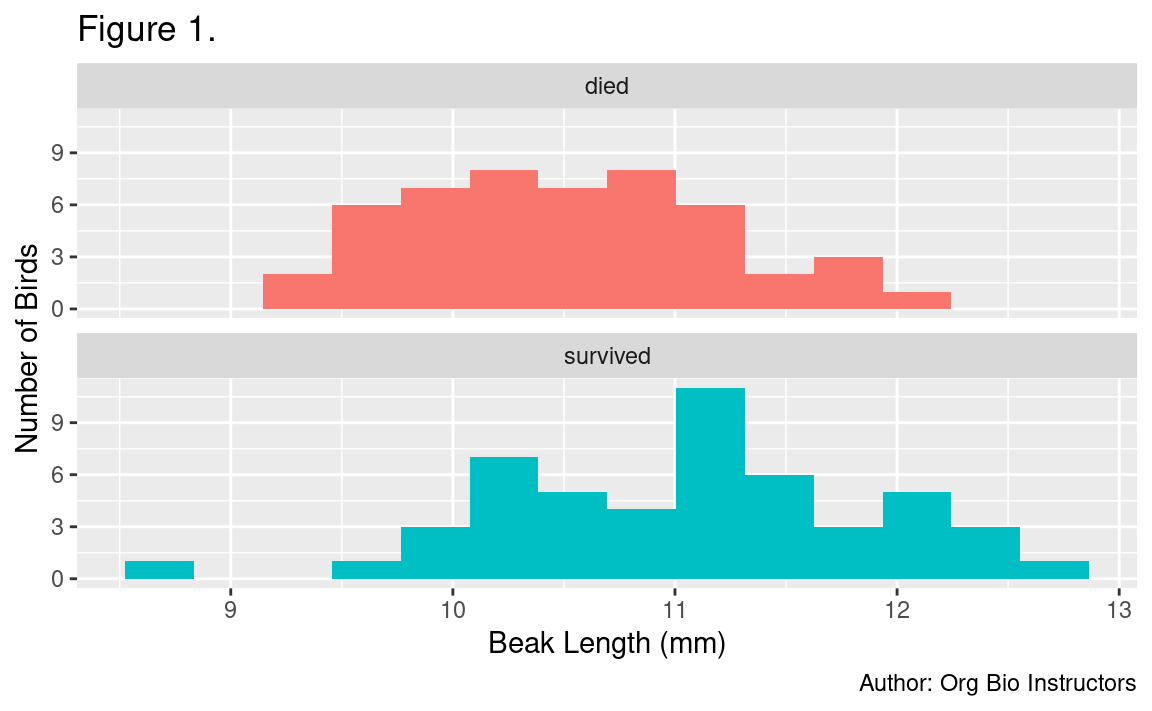
The last thing you might want to do is spruce up your plot by adding better axis labels and removing the redundant color legend. This is done with the labs() function and the :
# histogram of beak length, grouped by survival, with labels
ggplot(
data = finches_data, # use the finches dataset
mapping = aes(x = beak_length, # put beak length on the x axis
fill = outcome) # fill sets the color of the boxes
) +
geom_histogram(bins = 14) + # add the histogram, use 14 bins
facet_wrap(~ outcome, ncol = 1) + # outcome is the grouping variable
guides(fill = "none") + # don't show a legend for fill color
labs(
title = "Figure 1.", # this is the first figure
x = "Beak Length (mm)", # human-readable x-axis label
y = "Number of Birds", # human-readable y-axis label
caption = "Author: Org Bio Instructors" # so we know who made it
)
6.6.4 Write a figure legend
When sharing your figure with others, for example in a report or peer-reviewed journal article, you should always include a figure legend.
A good figure legend for this histogram would be:
Figure 1. Distribution of beak lengths among medium ground finches (Geospiza fortis) grouped by outcome during the 1977 drought on Daphne Major, Galapagos archipelago. Each sample consisted of 50 individuals. Birds were banded and measured during 1975-1977 and resighted in 1978.
6.6.5 Export your figure
Often when you complete a data analysis, you want to incorporate the figures you create into a report or manuscript. While YOU DO NOT NEED TO DO ANYTHING WITH THE FINCH FIGURES as part of your assignment today, it will be useful to know how to do it when you reach the Intraspecific Variation analysis later in the lab.
To put your figure in a Microsoft Word document, you must first save it as an image file on your computer. You can do this with ggsave(), which saves the most recent ggplot displayed.
In your finch script, paste this code below the code you used to create your histogram:
# save your most recent plot
ggsave("beak_length_histogram.png", # you choose a name for the file
width = 3.5, height = 3.5, # dimensions of saved file DO NOT CHANGE
units = "in") # units for the dimensions DO NOT CHANGEBe sure to put the name of the image file you will create inside quotation marks as above. It should end with “.png”, to indicate you want a PNG file. Here we have chosen a good width and height in inches (3.5” x 3.5”) so that the fonts are sized appropriately on the image.
After you run this code, look in Files tab in RStudio and you’ll see a file named beak_length_histogram.png now. You can click it to open the file in your computer’s default image viewer.
6.7 Summarize the data
6.7.1 Summarize by group
The following code will summarize the dataset by calculating the mean, standard deviation and standard error of the mean (SEM) of the beak_length variable.
Notice the use of the equations you learned in Part 2.
The group_by(outcome) function tells R to give us separate summaries for each outcome (died vs. survived).
# summarize ---------------------------------------------------------------
# summarize the dataset by outcome (survived vs. died)
beak_length_grouped_summary <-
finches_data %>%
group_by(outcome) %>%
summarize(
mean = mean(beak_length),
sample_size = n(),
standard_error = sd(beak_length) / sqrt(sample_size),
upper_conf_limit = mean + 1.96 * standard_error,
lower_conf_limit = mean - 1.96 * standard_error
) %>%
print() # print the results in the console#> # A tibble: 2 × 6
#> outcome mean sample_size standard_error upper_conf_limit lower_conf_limit
#> <chr> <dbl> <int> <dbl> <dbl> <dbl>
#> 1 died 10.5 50 0.0987 10.7 10.3
#> 2 survived 11.1 50 0.119 11.3 10.8The number 1.96 in the code above is derived from statistical theory. You will not need to change it for any of the analyses you do later.
6.7.2 Plot summarized data
To plot these means and confidence intervals, you can use a bar chart with the geom_col() and geom_errorbar() functions in ggplot:
# bar chart ---------------------------------------------------------------
# bar chart of mean beak lengths
ggplot(
data = beak_length_grouped_summary, # use summary data, not "finches_data"
mapping = aes(x = outcome, # survival on the x axis
y = mean, # mean beak length on the y axis
fill = outcome) # make died/survived different colors
) +
geom_col() + # add columns
geom_errorbar( # add error bars
mapping = aes(ymin = lower_conf_limit, # lower 95% confidence limit
ymax = upper_conf_limit), # upper 95% confidence limit
width = .3 # width of horizontal bars
) +
guides(fill = "none") + # don't show fill color legend
labs(
title = "Figure 2.", # this is the second figure
x = "Outcome", # human-readable x-axis label
y = "Beak Length (mm)", # human-readable y-axis label
caption = "Author: Org Bio Instructors" # so we know who made it
)
A good figure legend for this figure would be:
Figure 2. Mean beak length of medium ground finches (Geospiza fortis) that survived or died during the 1977 drought on Daphne Major, Galapagos archipelago. Each sample consisted of 50 individuals. Birds were banded and measured during 1975-1977 and resighted in 1978. Error bars represent 95% confidence intervals.
Export your figure:
# save the beak length bar chart
# note that the dimensions are different from the histograms above
ggsave("beak_length_bar_chart.png",
width = 2.5, height = 3.5, units = "in") # DO NOT CHANGE6.7.3 Compare means
You have visualized the different samples, and it looks like there is a difference in the means.
The question to ask is: could you have observed a difference as great as this if there is no real difference in the populations. Could you happen to have individuals with shorter beaks in one group simply due to an act of random samping? To answer that question, you need a statistical hypothesis test.
6.7.3.1 Conduct a t-test
A t-test is a statistical test that will tell you if the difference in means between two groups is likely due to chance (i.e. sampling error) or not.
The t-test function in R is t.test() and can be used like this:
# t-test ------------------------------------------------------------------
# perform a two-sample t-test assuming unequal variances
t.test(beak_length ~ outcome, data = finches_data)#>
#> Welch Two Sample t-test
#>
#> data: beak_length by outcome
#> t = -3.6335, df = 94.807, p-value = 0.0004539
#> alternative hypothesis: true difference in means between group died and group survived is not equal to 0
#> 95 percent confidence interval:
#> -0.8681443 -0.2546557
#> sample estimates:
#> mean in group died mean in group survived
#> 10.5122 11.0736The first part of the code above, beak_length ~ outcome, tells R to compare the mean beak lengths among the two outcomes (died or survived).
The second part, data = finches_data, tells R to use the finches dataset.
6.7.3.2 Interpret the Results
The two numbers to focus on here are the p-value and the 95% confidence interval.
The p-value tells you what proportion of the time you would expect to find a difference in population like these two if they were in fact the same. Scientists generally use a cutoff (or alpha level) of 0.05 to determine if a difference is “statistically significant”. In this case, a p-value of 0.0004539 is much lower than 0.05, so we can reject our null hypothesis and accept our alternative hypothesis that the true difference in means is not equal to 0.
As further evidence that the difference between the means is not equal to zero, you can see that the 95% confidence interval does not include zero.
To report the findings of a t-test, for example in the Results section of a report or scientific article, you would say:
Birds that survived the 1977 drought generally had longer beaks than those that died (Fig. 1). Mean beak lengths were 11.1 mm in survivors and 10.5 mm in non-survivors (Fig. 2). This difference was stastistically significant according to a Welch’s two-sample t-test assuming unequal variances (t=3.6335, df=94.807, p=0.0004539).